Abstract
Introduction: Plamotamab (XmAb13676) is a humanized bispecific antibody that binds both CD20 and CD3 to recruit cytotoxic T cells to kill CD20 expressing malignant cells. Interim results of an ongoing first-in-human (FIH), dose-escalation study (XmAb13676-01; NCT02924402) in subjects with relapsed/refractory (R/R) non-Hodgkin's lymphoma (NHL) are reported.
Methods: The study is an FIH, multi-center, open-label, phase 1, dose-escalation study in subjects with R/R NHL with a standard 3 + 3 design. The primary objectives are to determine safety, tolerability, and the maximum tolerated dose (MTD) or recommended dose of plamotamab. Secondary objectives include preliminary anti-tumor activity and pharmacokinetics/pharmacodynamics of plamotamab. This study has 3 Parts: Part A, escalating dose cohorts that establish an initial priming dose as part of repeated weekly infusions at a fixed, weight-based dose in a 28-day cycle; Part B, with a dosing schedule consisting of a priming dose on Cycle 1 Day 1, established in Part A, followed by step-up dosing (SUD) on subsequent weeks; and Part C is an SUD regimen with flat and less frequent dosing. Cytokine release syndrome (CRS) prophylaxis with dexamethasone, antihistamine, and acetaminophen was mandated prior to each administration of plamotamab. Treatment was continued for 2 cycles or longer if there was evidence of therapeutic benefit.
Results: At data cut-off (01Jul2021), 80 subjects with NHL have been treated.
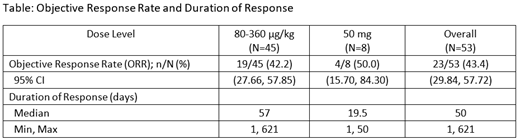
Subjects had a median age of 62 years (range 32-89), a median of 4 prior therapies (range 1-10), and had been diagnosed a median of 28 months (range 6-353) prior to treatment in the study. The most common treatment-related adverse event was CRS. Overall, 50 subjects (62.5%) experienced CRS, with 4 (5.0%) experiencing Grade ≥ 3 CRS. No related neurotoxicity >Grade 2 has been observed. Treatment responses for NHL were assessed by the Lugano criteria or International Working Group criteria for Waldenström Macroglobulinemia. There have been 23 objective responses (43.4% overall response rate [ORR]) and 13 (38.2%) at doses of ≥ 80 µg/kg or flat dosing in all evaluable subjects with NHL and diffuse large cell B-cell lymphoma, respectively. In the efficacy-evaluable, follicular lymphoma population, at doses of 80-360 µg/kg or flat dosing, objective responses were observed in 8/10 (80%) subjects. After implementation of flat dosing, as opposed to weight-based dosing, a 50% ORR (4/8 evaluable subjects) has been observed in NHL subjects in that cohort. An MTD has not been reached, and dose escalation is ongoing. Overall, 4 out of 16 (25%) evaluable subjects with prior CAR-T therapy responded to plamotamab.
Conclusions: Plamotamab demonstrated evidence of clinical activity in heavily pretreated subjects with R/R NHL. CRS was generally manageable with premedication. The study is ongoing with further optimization of dose and schedule.
Patel: Kite Pharma: Consultancy, Speakers Bureau; Genentech: Consultancy; Morphosys: Consultancy; Bristol Myers Squibb: Consultancy, Speakers Bureau; BeiGene: Consultancy; Janssen: Consultancy; AstraZeneca: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics: Consultancy; Abbvie: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; MEI Pharma: Consultancy; ADC Therapeutics: Consultancy; Lilly: Consultancy. Michot: GSK: Honoraria; Celgene: Honoraria; MSD: Consultancy, Honoraria; Innate Pharma: Research Funding; Incyte: Research Funding; H3 biomedecine: Research Funding; GSK: Consultancy, Honoraria, Research Funding; Genentech: Research Funding; Gamamabs: Research Funding; Forma: Research Funding; Exelixis: Research Funding; Eos: Research Funding; Eisai: Research Funding; Debiopharm: Research Funding; Daiichi Sankyo,: Research Funding; Clovis: Research Funding; Chugai: Research Funding; Boeringer Ingelheim: Research Funding; Celgene: Research Funding; Blueprint: Research Funding; Beigene: Research Funding; Bayer: Research Funding; Argen-x: Research Funding; Amgen: Research Funding; Agios: Research Funding; Aduro: Research Funding; Abbvie: Research Funding; ASTEX: Research Funding, Speakers Bureau; Astra Zeneca: Honoraria, Research Funding; Roche: Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. Chanan-Khan: Alpha2 Pharmaceuticals, NonoDev, Starton: Current holder of stock options in a privately-held company; Ascentage: Research Funding; Cellectar: Current equity holder in publicly-traded company; Alpha2 Pharmaceuticals: Patents & Royalties: Tabi; Ascentage, Starton, Cellectar, NonoDev, Alpha2 Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; BeiGene, Jansen, Ascentage: Honoraria; BieGene, Jansen, Ascentage: Consultancy. Ghesquieres: Janssen: Honoraria; Mundipharma: Consultancy, Honoraria; Roche: Consultancy; Celgene: Consultancy, Honoraria; Gilead Science: Consultancy, Honoraria. Byrd: Vincerx Pharmaceuticals: Current equity holder in publicly-traded company, Membership on an entity's Board of Directors or advisory committees; Novartis, Trillium, Astellas, AstraZeneca, Pharmacyclics, Syndax: Consultancy, Honoraria; Newave: Membership on an entity's Board of Directors or advisory committees. Cartron: Roche, Celgene-BMS: Consultancy; Danofi, Gilead, Novartis, Jansen, Roche, Celgene-BMS, Abbvie, Takeda: Honoraria. Portell: Acerta/AstraZeneca: Research Funding; Xencor: Research Funding; SeaGen: Research Funding; Targeted Oncology: Honoraria; Aptitude Health: Honoraria; Abbvie: Research Funding; Pharmacyclics: Honoraria; Kite: Honoraria, Research Funding; Merck: Honoraria, Research Funding; BeiGene: Honoraria, Research Funding; Morphosys: Honoraria; TG Therapeutics: Honoraria, Research Funding; Genentech: Research Funding; VelosBio: Research Funding. Solh: Jazz Pharmaceuticals: Consultancy; Partner Therapeutics: Research Funding; BMS: Consultancy; ADCT Therapeutics: Consultancy, Research Funding. Wierda: Loxo Oncology, Inc.: Research Funding; GSK/Novartis: Research Funding; Pharmacyclics LLC, an AbbVie Company: Research Funding; Miragen: Research Funding; Sunesis: Research Funding; Gilead Sciences: Research Funding; KITE Pharma: Research Funding; Genentech: Research Funding; Janssen: Research Funding; Juno Therapeutics: Research Funding; Acerta Pharma Inc.: Research Funding; Xencor: Research Funding; AstraZeneca: Research Funding; Cyclacel: Research Funding; Oncternal Therapeutics, Inc.: Research Funding; Karyopharm: Research Funding; Genzyme Corporation: Consultancy; AbbVie: Research Funding. Johnson: Xencor: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months, Ended employment in the past 24 months. Garcha: Xencor: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company; Promedim: Ended employment in the past 24 months. Ribrag: AstraZeneca: Membership on an entity's Board of Directors or advisory committees; GSK: Research Funding; MSD Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees; Infinity Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy, Membership on an entity's Board of Directors or advisory committees; Argen-X: Research Funding; Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees; Astex Pharmaceuticals: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees; Nanostring: Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; PharmaMar: Honoraria, Membership on an entity's Board of Directors or advisory committees; Epizyme: Honoraria, Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees. Liebowitz: Xencor: Current Employment, Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months. Phillips: Incyte: Consultancy, Other: received travel expenses from Incyte, Research Funding; ADCT, BeiGene, Bristol Myers Squibb, Cardinal Health, Incyte, Karyopharm, Morphosys, Pharmacyclics, Seattle Genetics: Consultancy; AbbVie: Consultancy, Research Funding; AstraZeneca: Consultancy; BMS: Consultancy, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal